12 Which of the Following Is Not a Reducing Agent
Not as much as that. Cl 2 is the oxidizing agent e.
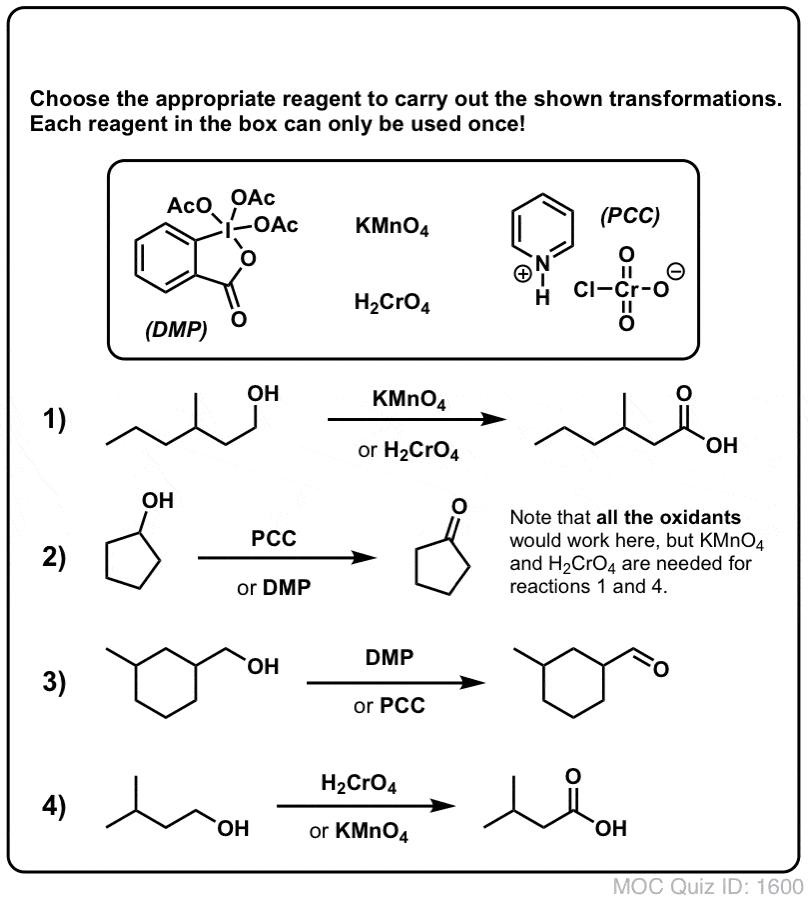
Alcohol Oxidation Strong Weak Oxidants Master Organic Chemistry
However Lithium Li is a stronger reducing agent than Na due to greater hydration energy.
. Benzoyl peroxide used in treatment of acne is A. Molecules and ions which contain relatively electropositive elements which have low oxidation numbers are also good reducing agents. Which of the following is not an antioxidant.
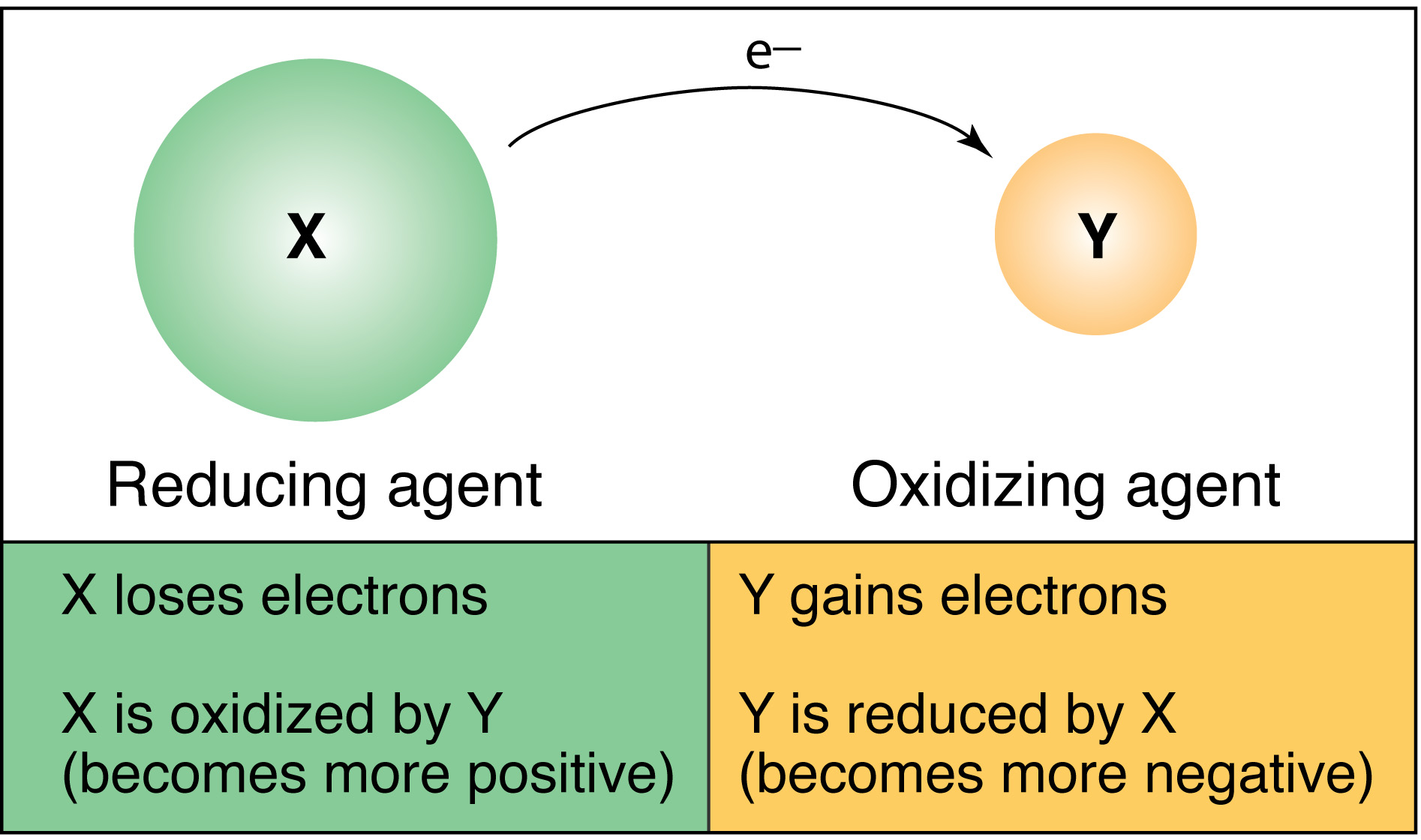
D Oxygen is reduced from a state of 0 to -2. Which of the following is a reducing agent A. A reducing agent loses electrons.
C in CO_ 2 is. NO_ 2 Answer. Which is maximum So it does not undergo oxidation and hence it is not a reducing agent.
The reducing agent reduces some part of another item. A species that is in an oxidation state other than its highest possible oxidation state can act as a reducing agent. Cr 2 is the strongest reducing agent.
Carbon is oxidized from 2 state to 4. A reducing agent is a substance that adds _____ to a chemical compound or subtracts oxygen from the compound. Similarly the weaker the oxidizing agent than the more strong is the corresponding reducing agent as shown in the figure below.
So hydrogen is the reducing agent. The equation is fully balanced. Poorest reducing agent among following is.
The reducing character increases from Na to Cs. It has a d4 configuration. Sodium hydrogen and lithium are examples of strong oxidizing agents.
Au is the reducing agent. Cr2 Is A Strong Reducing Agent Whereas Mn2 Is Not Cr24 Mn25 The reducing agent is a compound which itself gets oxidized. Disproportionation A substance called an ______ agent has the potential to cause oxidation in another substance but a substance with the potential to cause reduction in another substance is an ______ agent.
Among the elements low electronegativity is characteristic of good reducing agents. Learn vocabulary terms and more with flashcards games and other study tools. We can know the strength of reducing agents by electrochemical series as well.
We also know that the weaker an acid then stronger is the conjugate base. An element that acts as a reducing agent must have low ionization energy. Alkali metals act as strong reducing agents as their ionization energy values are low.
More than one statement is not correct. Which of the following species can act as reducing agent. All have same reducing strength.
Fluorine gas is known to be a strong oxidizing agent and whereas F- is said to be a weak reducing agent. Which one of the following items does not characterize an oxidizing agent. The hydroxide does not change any of its reduction status.
While weak reducing agents cannot lose electrons easily. An oxidizing agent D. Cr 2 better reducing agent but Mn3 is a better oxidizing agent because.
An example of a good oxidizing agent is an alkai metal such as Na. 1CoCO4- 2MnCO6 3MnCO5 4CrCO6 Practice questions MCQs Past Year Questions PYQs NCERT Questions Question Bank Class 11 and Class 12 Questions NCERT Exemplar Questions and PDF Questions with answers solutions explanations NCERT reference and difficulty level. Fluorine chlorine iron etc.
Essentially the reducing agent becomes. A good reducing agent must be able to donate electrons readily meaning it must not have a high electronegativity. The process in which a substance acts as both an oxidizing agent and a reducing agent in the same reaction is called _____.
The oxidation number of a reducing agent increases. Which one of the following items does not characterize a reducing agent. Which of the following is NOT a physical mixture.
The oxidizing agent oxidizes some part of an item. A good reducing agent is a metal in a high oxidation state such as Mn 7. Are weak reducing agents.
A good reducing agent is. A reducing agent causes another species to be reduced. So carbon monozide is reducing agent.
Therefore I N a and FeX 2 all will act as reducing agents. Which one of the following items does not characterize a reducing agent.
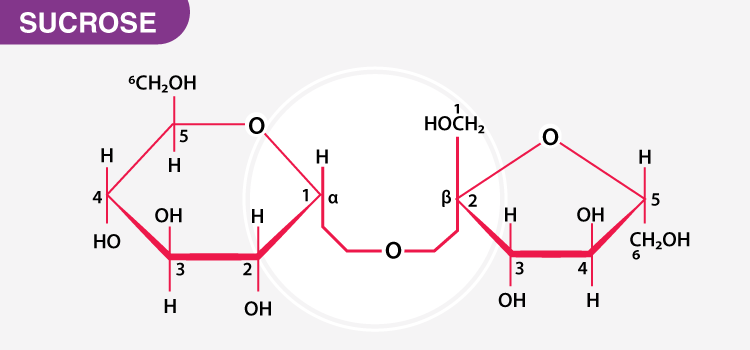
What Are Five Examples Of Non Reducing Sugars Chemistry Question

Difference Between Reducing Sugar And Starch Compare The Difference Between Similar Terms
11 19 Common Reducing Agents Chemistry Libretexts

Reducing Agent Reductant Definition Examples With Videos

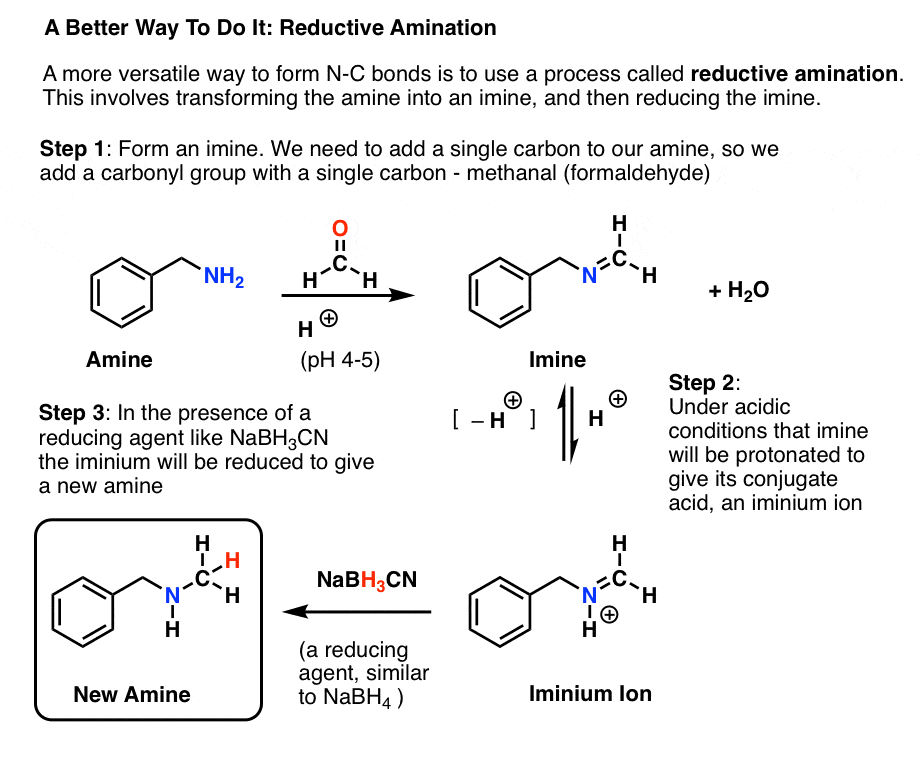
Reductive Amination And How It Works Master Organic Chemistry

Reducing Agents An Overview Sciencedirect Topics

How To Find The Oxidizing And Reducing Agent Youtube
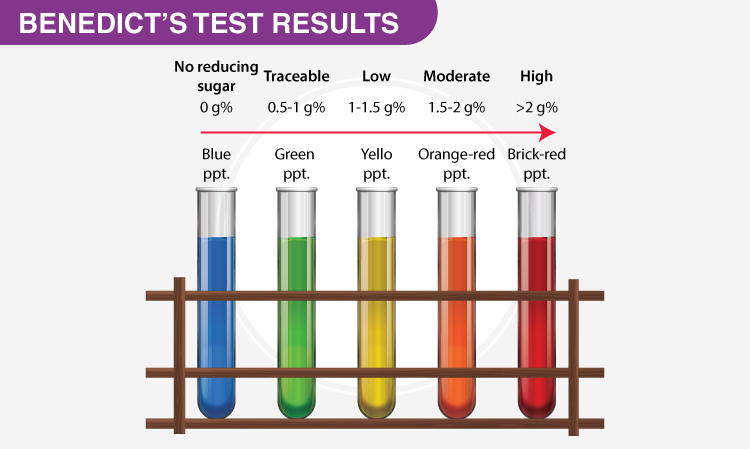
What Are Five Examples Of Non Reducing Sugars Chemistry Question

How To Find The Oxidizing And Reducing Agent Youtube
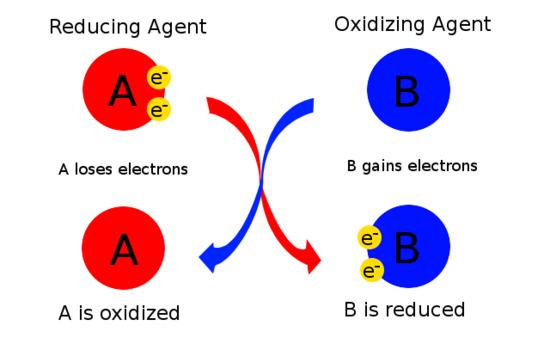
8 2 Oxidizing And Reducing Agents Chemistry Libretexts
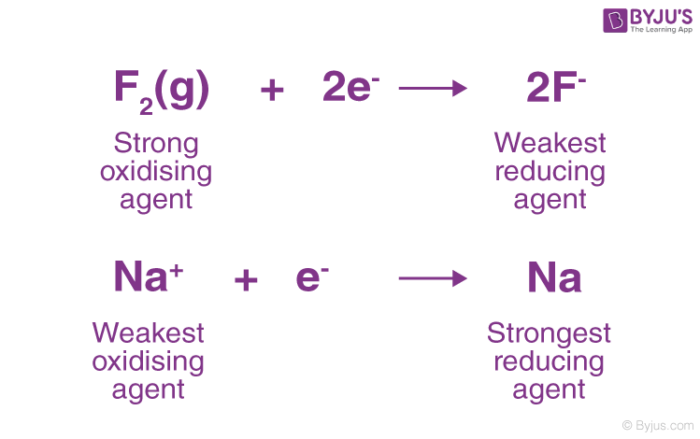
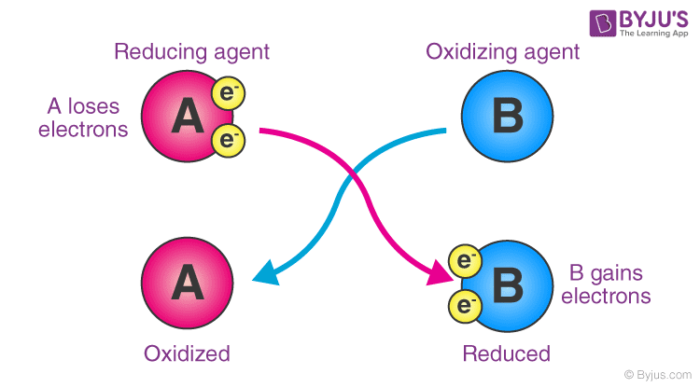
Reducing Agent Reductant Definition Examples With Videos
Oxidizing Agent Definition Properties Examples Applications

Organic Chemistry Preference For Tin Or Iron In The Reduction Of Nitrobenzene Chemistry Stack Exchange

Oxidizing And Reducing Agents Hindi Youtube

Oxidizing Agents And Reducing Agents Youtube
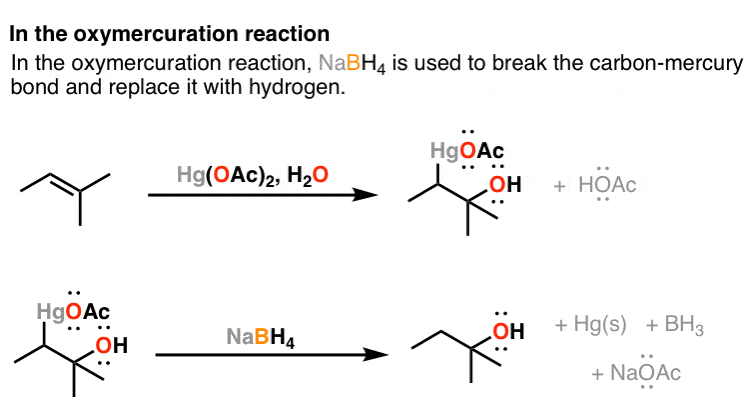
Sodium Borohydride Nabh4 As A Reagent In Organic Chemistry

Disproportionation Definition Examples Video Lesson Transcript Study Com

Comments
Post a Comment